Active Reading Section: the Cell Cycle Answer Key
Prison cell Reproduction
52 Control of the Cell Cycle
Learning Objectives
Past the end of this section, you will be able to do the following:
- Empathize how the jail cell wheel is controlled past mechanisms that are both internal and external to the prison cell
- Explain how the three internal "command checkpoints" occur at the cease of Gi, at the G2/Thousand transition, and during metaphase
- Describe the molecules that control the cell cycle through positive and negative regulation
The length of the cell wheel is highly variable, even within the cells of a single organism. In humans, the frequency of cell turnover ranges from a few hours in early embryonic development, to an average of two to five days for epithelial cells, and to an entire homo lifetime spent in M0 past specialized cells, such as cortical neurons or cardiac muscle cells.
In that location is as well variation in the fourth dimension that a cell spends in each stage of the cell cycle. When rapidly dividing mammalian cells are grown in a culture (outside the body nether optimal growing conditions), the length of the cell cycle is virtually 24 hours. In rapidly dividing human cells with a 24-hour jail cell cycle, the G1 stage lasts approximately nine hours, the South phase lasts 10 hours, the Gii stage lasts about iv and half hours, and the M phase lasts approximately half hour. By comparison, in fertilized eggs (and early embryos) of fruit flies, the cell bicycle is completed in about 8 minutes. This is because the nucleus of the fertilized egg divides many times by mitosis but does not go through cytokinesis until a multinucleate "zygote" has been produced, with many nuclei located forth the periphery of the prison cell membrane, thereby shortening the time of the cell partitioning cycle. The timing of events in the prison cell cycle of both "invertebrates" and "vertebrates" is controlled by mechanisms that are both internal and external to the jail cell.
Regulation of the Cell Cycle past External Events
Both the initiation and inhibition of cell division are triggered by events external to the cell when it is about to begin the replication procedure. An event may be every bit simple every bit the death of nearby cells or as sweeping every bit the release of growth-promoting hormones, such as human growth hormone (HGH or hGH). A lack of HGH tin inhibit jail cell division, resulting in dwarfism, whereas likewise much HGH can result in gigantism. Crowding of cells tin can as well inhibit jail cell segmentation. In contrast, a cistron that tin can initiate cell partition is the size of the cell: Equally a cell grows, it becomes physiologically inefficient due to its decreasing surface-to-book ratio. The solution to this problem is to dissever.
Whatever the source of the message, the jail cell receives the indicate, and a series of events inside the jail cell allows it to proceed into interphase. Moving forward from this initiation point, every parameter required during each cell bicycle phase must exist met or the cycle cannot progress.
Regulation at Internal Checkpoints
Information technology is essential that the daughter cells produced be exact duplicates of the parent prison cell. Mistakes in the duplication or distribution of the chromosomes pb to mutations that may be passed forward to every new prison cell produced from an abnormal cell. To prevent a compromised cell from standing to dissever, there are internal control mechanisms that operate at three main prison cell-cycle checkpoints: A checkpoint is one of several points in the eukaryotic jail cell cycle at which the progression of a cell to the next stage in the bicycle can exist halted until conditions are favorable. These checkpoints occur near the finish of Gone, at the 1000ii/M transition, and during metaphase ((Figure)).
The cell cycle is controlled at iii checkpoints. The integrity of the DNA is assessed at the Gone checkpoint. Proper chromosome duplication is assessed at the G2 checkpoint. Attachment of each kinetochore to a spindle fiber is assessed at the G checkpoint.
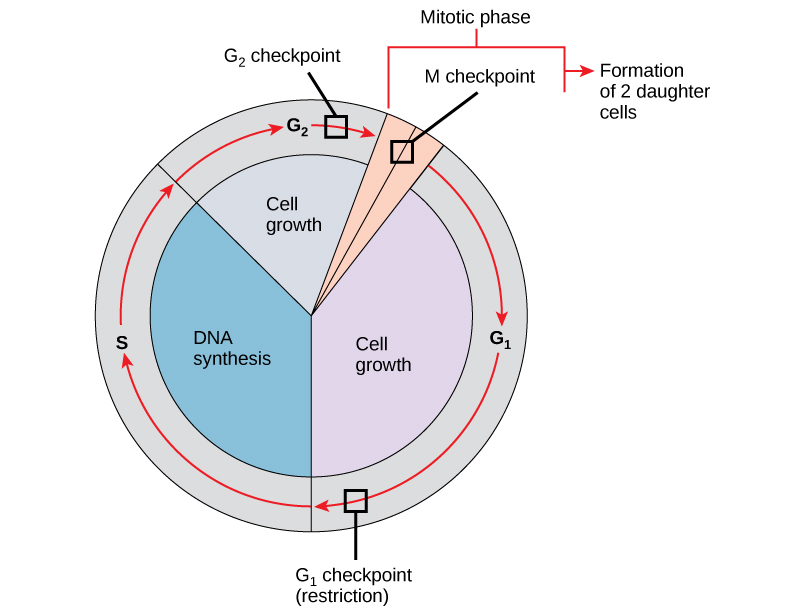
The G1 Checkpoint
The Kone checkpoint determines whether all weather condition are favorable for prison cell division to proceed. The 10001 checkpoint, also called the restriction point (in yeast), is a point at which the prison cell irreversibly commits to the prison cell division process. External influences, such as growth factors, play a large function in carrying the cell past the G1 checkpoint. In addition to adequate reserves and prison cell size, there is a check for genomic DNA damage at the Grandone checkpoint. A prison cell that does non meet all the requirements will not be allowed to progress into the S stage. The jail cell can halt the bicycle and attempt to remedy the problematic condition, or the cell can advance into G0 and await further signals when weather condition better.
The One thousand2 Checkpoint
The Gii checkpoint bars entry into the mitotic phase if certain atmospheric condition are not met. As at the Thousandone checkpoint, cell size and protein reserves are assessed. However, the most important role of the K2 checkpoint is to ensure that all of the chromosomes accept been replicated and that the replicated Deoxyribonucleic acid is not damaged. If the checkpoint mechanisms detect problems with the Dna, the cell bicycle is halted, and the jail cell attempts to either complete Dna replication or repair the damaged DNA.
The Chiliad Checkpoint
The K checkpoint occurs most the end of the metaphase stage of karyokinesis. The Chiliad checkpoint is as well known every bit the spindle checkpoint, considering it determines whether all the sister chromatids are correctly attached to the spindle microtubules. Because the separation of the sister chromatids during anaphase is an irreversible step, the cycle volition not go along until the kinetochores of each pair of sister chromatids are firmly anchored to at least two spindle fibers arising from contrary poles of the prison cell.
Link to Learning
Sentry what occurs at the Gi, Kii, and M checkpoints by visiting this website to run across an animation of the jail cell bicycle.
Regulator Molecules of the Cell Cycle
In addition to the internally controlled checkpoints, in that location are ii groups of intracellular molecules that regulate the jail cell cycle. These regulatory molecules either promote progress of the cell to the next stage (positive regulation) or halt the cycle (negative regulation). Regulator molecules may human action individually, or they can influence the activity or production of other regulatory proteins. Therefore, the failure of a single regulator may have almost no effect on the cell bike, specially if more than one machinery controls the same issue. Withal, the effect of a deficient or non-operation regulator can be wide-ranging and mayhap fatal to the cell if multiple processes are affected.
Positive Regulation of the Jail cell Cycle
Two groups of proteins, called cyclins and cyclin-dependent kinases (Cdks), are termed positive regulators. They are responsible for the progress of the jail cell through the various checkpoints. The levels of the four cyclin proteins fluctuate throughout the prison cell cycle in a predictable pattern ((Figure)). Increases in the concentration of cyclin proteins are triggered by both external and internal signals. After the cell moves to the adjacent phase of the cell bicycle, the cyclins that were active in the previous phase are degraded by cytoplasmic enzymes, as shown in (Figure) below.
The concentrations of cyclin proteins change throughout the cell cycle. At that place is a directly correlation between cyclin aggregating and the three major cell-bike checkpoints. Also notation the sharp reject of cyclin levels following each checkpoint (the transition between phases of the cell wheel), as cyclin is degraded by cytoplasmic enzymes. (credit: modification of work by "WikiMiMa"/Wikimedia Commons)
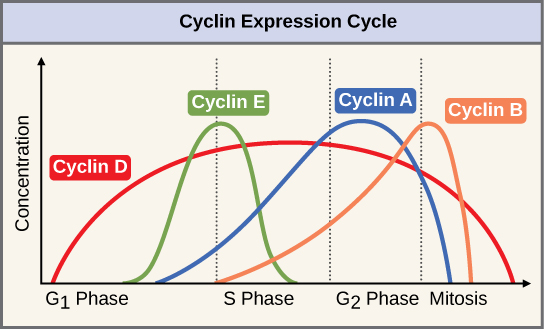
Cyclins regulate the cell wheel only when they are tightly bound to Cdks. To be fully agile, the Cdk/cyclin complex must also be phosphorylated in specific locations to activate the complex. Like all kinases, Cdks are enzymes (kinases) that in plow phosphorylate other proteins. Phosphorylation activates the poly peptide past irresolute its shape. The proteins phosphorylated by Cdks are involved in advancing the cell to the next phase. ((Figure)). The levels of Cdk proteins are relatively stable throughout the cell cycle; however, the concentrations of cyclin fluctuate and decide when Cdk/cyclin complexes form. The different cyclins and Cdks bind at specific points in the cell cycle and thus regulate different checkpoints.
Cyclin-dependent kinases (Cdks) are protein kinases that, when fully activated, can phosphorylate and thus actuate other proteins that advance the prison cell cycle by a checkpoint. To become fully activated, a Cdk must demark to a cyclin poly peptide and so be phosphorylated by another kinase.
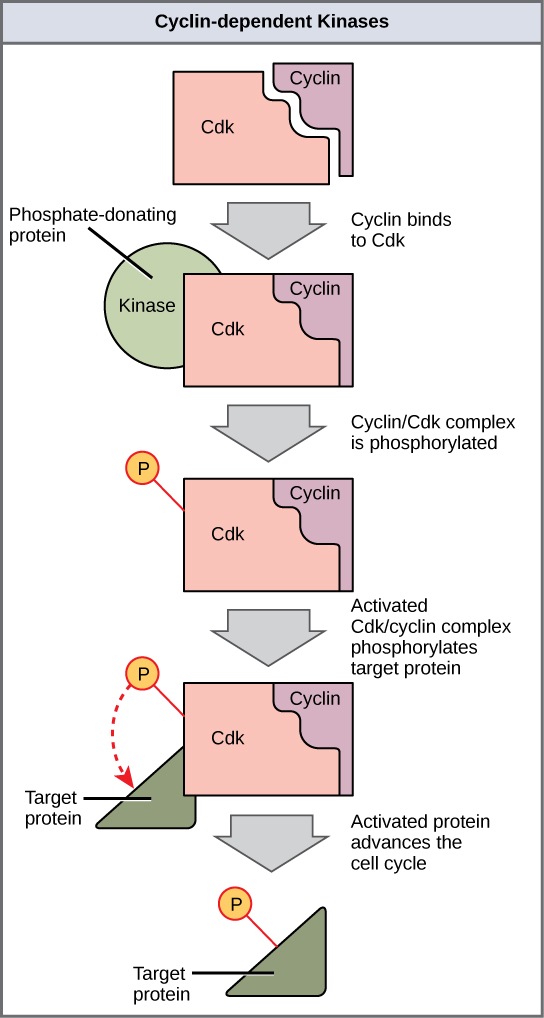
Because the circadian fluctuations of cyclin levels are largely based on the timing of the jail cell cycle and non on specific events, regulation of the prison cell cycle unremarkably occurs past either the Cdk molecules alone or the Cdk/cyclin complexes. Without a specific concentration of fully activated cyclin/Cdk complexes, the cell cycle cannot keep through the checkpoints.
Although the cyclins are the principal regulatory molecules that determine the forward momentum of the prison cell cycle, there are several other mechanisms that fine-tune the progress of the cycle with negative, rather than positive, effects. These mechanisms essentially block the progression of the cell cycle until problematic conditions are resolved. Molecules that prevent the full activation of Cdks are called Cdk inhibitors. Many of these inhibitor molecules directly or indirectly monitor a detail cell-cycle event. The cake placed on Cdks by inhibitor molecules will not be removed until the specific event that the inhibitor monitors is completed.
Negative Regulation of the Jail cell Bike
The second group of jail cell-cycle regulatory molecules are negative regulators, which cease the cell wheel. Call back that in positive regulation, agile molecules crusade the bicycle to progress.
The best understood negative regulatory molecules are retinoblastoma protein (Rb), p53, and p21. Retinoblastoma proteins are a group of tumor-suppressor proteins common in many cells. Nosotros should note here that the 53 and 21 designations refer to the functional molecular masses of the proteins (p) in kilodaltons (a dalton is equal to an atomic mass unit of measurement, which is equal to ane proton or i neutron or 1 yard/mol). Much of what is known about cell-cycle regulation comes from inquiry conducted with cells that take lost regulatory command. All iii of these regulatory proteins were discovered to be damaged or non-functional in cells that had begun to replicate uncontrollably (i.e., became cancerous). In each instance, the chief crusade of the unchecked progress through the cell wheel was a faulty copy of the regulatory protein.
Rb, p53, and p21 human action primarily at the G1 checkpoint. p53 is a multi-functional protein that has a major impact on the commitment of a jail cell to partition because information technology acts when there is damaged DNA in cells that are undergoing the preparatory processes during Gone. If damaged DNA is detected, p53 halts the cell wheel and and so recruits specific enzymes to repair the DNA. If the Dna cannot exist repaired, p53 can trigger apoptosis, or cell suicide, to prevent the duplication of damaged chromosomes. As p53 levels rising, the production of p21 is triggered. p21 enforces the halt in the cycle dictated by p53 by bounden to and inhibiting the action of the Cdk/cyclin complexes. As a cell is exposed to more stress, college levels of p53 and p21 accumulate, making it less likely that the cell will move into the South phase.
Rb, which largely monitors cell size, exerts its regulatory influence on other positive regulator proteins. In the active, dephosphorylated state, Rb binds to proteins called transcription factors, most commonly, E2F ((Figure)). Transcription factors "turn on" specific genes, assuasive the production of proteins encoded by that gene. When Rb is bound to E2F, production of proteins necessary for the G1/S transition is blocked. As the cell increases in size, Rb is slowly phosphorylated until information technology becomes inactivated. Rb releases E2F, which tin now plow on the gene that produces the transition protein, and this particular block is removed. For the cell to motion past each of the checkpoints, all positive regulators must be "turned on," and all negative regulators must exist "turned off."
Visual Connection
Rb halts the cell cycle and releases its hold in response to prison cell growth.
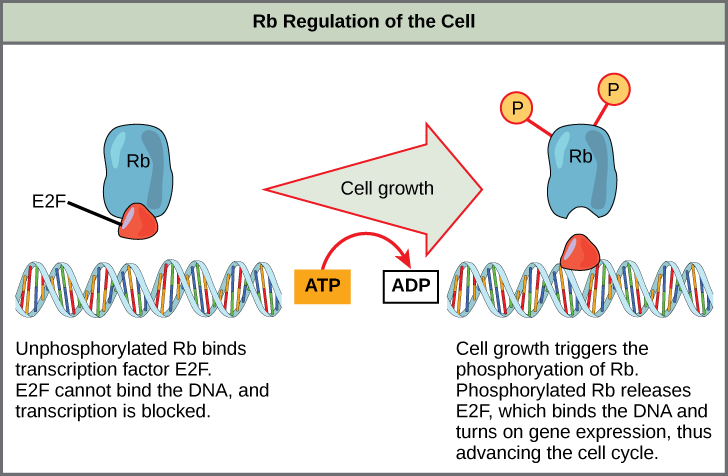
Rb and other proteins that negatively regulate the jail cell bicycle are sometimes chosen tumor suppressors. Why do yous call back the name tumor suppressor might be advisable for these proteins?
Section Summary
Each step of the prison cell cycle is monitored by internal controls called checkpoints. At that place are three major checkpoints in the cell cycle: one near the terminate of Thousand1, a 2nd at the Grand2/M transition, and the third during metaphase. Positive regulator molecules permit the cell bike to advance to the next stage of prison cell division. Negative regulator molecules monitor cellular conditions and can halt the bike until specific requirements are met.
Visual Connection Questions
(Figure) Rb and other proteins that negatively regulate the cell cycle are sometimes called tumor suppressors. Why do you recollect the name tumor suppressor might exist appropriate for these proteins?
(Figure) Rb and other negative regulatory proteins command cell division and therefore preclude the formation of tumors. Mutations that prevent these proteins from carrying out their function can result in cancer.
Review Questions
At which of the prison cell-bicycle checkpoints do external forces have the greatest influence?
- G1 checkpoint
- M2 checkpoint
- M checkpoint
- G0 checkpoint
A
What is the master prerequisite for clearance at the Gtwo checkpoint?
- prison cell has reached a sufficient size
- an adequate stockpile of nucleotides
- accurate and consummate Dna replication
- proper zipper of mitotic spindle fibers to kinetochores
C
If the Chiliad checkpoint is not cleared, what stage of mitosis volition be blocked?
- prophase
- prometaphase
- metaphase
- anaphase
D
Which protein is a positive regulator that phosphorylates other proteins when activated?
- p53
- retinoblastoma protein (Rb)
- cyclin
- cyclin-dependent kinase (Cdk)
D
Many of the negative regulator proteins of the prison cell cycle were discovered in what blazon of cells?
- gametes
- cells in Yard0
- cancer cells
- stalk cells
C
Which negative regulatory molecule can trigger jail cell suicide (apoptosis) if vital cell cycle events practice non occur?
- p53
- p21
- retinoblastoma protein (Rb)
- cyclin-dependent kinase (Cdk)
A
Critical Thinking Questions
Describe the general conditions that must be met at each of the iii main cell-bike checkpoints.
The Thousand1 checkpoint monitors adequate prison cell growth, the land of the genomic Deoxyribonucleic acid, adequate stores of energy, and materials for S stage. At the Gii checkpoint, DNA is checked to ensure that all chromosomes were duplicated and that at that place are no mistakes in newly synthesized Dna. Additionally, jail cell size and energy reserves are evaluated. The 1000 checkpoint confirms the correct attachment of the mitotic spindle fibers to the kinetochores.
Compare and contrast the roles of the positive prison cell-cycle regulators negative regulators.
Positive cell regulators such every bit cyclin and Cdk perform tasks that advance the cell bike to the next stage. Negative regulators such as Rb, p53, and p21 cake the progression of the jail cell bicycle until sure events have occurred.
What steps are necessary for Cdk to go fully active?
Cdk must demark to a cyclin, and it must be phosphorylated in the correct position to become fully active.
Rb is a negative regulator that blocks the cell cycle at the Thoui checkpoint until the cell achieves a requisite size. What molecular mechanism does Rb employ to halt the jail cell cycle?
Rb is active when it is dephosphorylated. In this state, Rb binds to E2F, which is a transcription factor required for the transcription and eventual translation of molecules required for the Grand1/S transition. E2F cannot transcribe sure genes when it is leap to Rb. As the prison cell increases in size, Rb becomes phosphorylated, inactivated, and releases E2F. E2F can then promote the transcription of the genes it controls, and the transition proteins volition be produced.
Glossary
- jail cell-cycle checkpoint
- machinery that monitors the preparedness of a eukaryotic prison cell to advance through the various cell-cycle stages
- cyclin
- one of a group of proteins that human action in conjunction with cyclin-dependent kinases to help regulate the jail cell cycle by phosphorylating fundamental proteins; the concentrations of cyclins fluctuate throughout the cell cycle
- cyclin-dependent kinase (Cdk)
- one of a group of poly peptide kinases that helps to regulate the cell cycle when bound to cyclin; it functions to phosphorylate other proteins that are either activated or inactivated past phosphorylation
- p21
- cell-cycle regulatory protein that inhibits the jail cell cycle; its levels are controlled past p53
- p53
- jail cell-cycle regulatory poly peptide that regulates cell growth and monitors Dna damage; it halts the progression of the cell cycle in cases of DNA impairment and may induce apoptosis
- retinoblastoma poly peptide (Rb)
- regulatory molecule that exhibits negative furnishings on the prison cell cycle past interacting with a transcription factor (E2F)
Source: https://opentextbc.ca/biology2eopenstax/chapter/control-of-the-cell-cycle/